What is Benzene?
Benzene is one of the most important organic compounds with the chemical formula C6H6. Benzene is the parent compound of the various aromatic compound.
Benzene is the simplest organic, aromatic hydrocarbon. Benzene is one of the elementary petrochemicals and a natural constituent of crude oil. It has a gasoline-like odour and is a colourless liquid. Benzene is highly toxic and carcinogenic in nature. It is primarily used in the production of polystyrene.
Benzene is a naturally occurring substance produced by volcanoes and forest fires and present in many plants and animals, but benzene is also a major industrial chemical made from coal and oil. As a pure chemical, benzene is a clear, colourless liquid. In industry, benzene is used to make other chemicals as well as some types of plastics, detergents, and pesticides. It is also a component of gasoline.
Discovery of Benzene
The word benzene derives historically from gum benzoin, sometimes called ‘Benjamin’. Gum benzoin was known as an aromatic resin. Michael Faraday, an English scientist first discovered Benzene in illuminating gas. The name benzene was given by German Chemist Mitscherich in 1833. The cyclic structure of benzene remained a mystery until 1865 when German professor August Kekule elucidated it when he dreamt of a snake biting its own tail.
However, Kekule did not discover the presence of interactions between the double bonds. American professor Linus Pauling proposed that benzene exhibited a hybrid structure composed of delocalized electrons. This was the refinement of Kekule’s discovery. Benzene has a somewhat pleasant, sweet smell, however it is carcinogenic.
Recommended Videos
Aromaticity Introduction

Aromaticity

Benzene Characteristics
Structure of Benzene
The structure of benzene has been of interest since its discovery. Benzene is a cyclic hydrocarbon (chemical formula: C6H6), i.e., each carbon atom in benzene is arranged in a six-membered ring and is bonded to only one hydrogen atom. According to molecular orbital theory, benzene ring involves the formation of three delocalized π – orbitals spanning all six carbon atoms, while the valence bond theory describes two stable resonance structures for the ring.
Properties of Benzene
The various properties of benzene are mentioned below:
- Benzene is immiscible in water but soluble in organic solvents.
- It is a colourless liquid and has an aromatic odour.
- It has a density of 0.87g cm-3. It is lighter than water.
- Benzene has a moderate boiling point and a high melting point. (Boiling point: 80.5°C, Melting point: 5.5°C)
- Benzene shows resonance.
- It is highly inflammable and burns with a sooty flame.
Resonance of Benzene
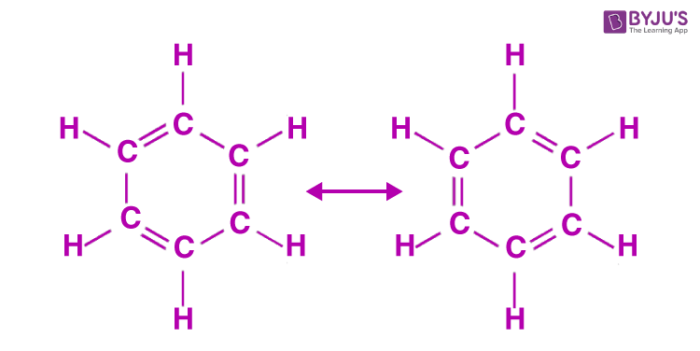
The oscillating double bonds in the benzene ring are explained with the help of resonance structures as per valence bond theory. All the carbon atoms in the benzene ring are sp2 hybridized. One of the two sp2 hybridized orbitals of one atom overlaps with the sp2 orbital of adjacent carbon atom forming six C-C sigma bonds. Other left sp2 hybridized orbitals combine with s orbital of hydrogen to form six C-H sigma bonds. Remaining unhybridized p orbitals of carbon atoms form π bonds with adjacent carbon atoms by lateral overlap.
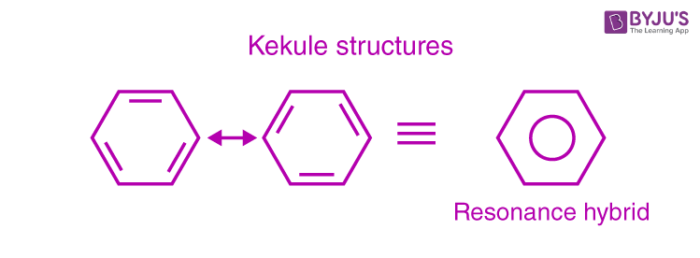
This explains an equal possibility for the formation of C1 –C2, C3 – C4, C5 – C6 π bonds or C2 – C3, C4 – C5, C6-C1 π bonds. The hybrid structure is represented by inserting a circle in the ring as shown below in the figure. Hence, it explains the formation of two resonance structures proposed by Kekule.
Aromaticity of benzene
Benzene is an aromatic compound, as the C-C bonds formed in the ring are not exactly single or double, rather they are of intermediate length. Aromatic compounds are divided into two categories: benzenoids (one containing benzene ring) and non-benzenoids (those not containing benzene ring), provided they follow Huckel rule. According to Huckel rule, for a ring to be aromatic it should have the following property:
- Planarity
- Complete delocalization of the π electrons in the ring
- Presence of (4n + 2) π electrons in the ring where n is an integer (n = 0, 1, 2, . . .)
Uses of Benzene
Benzene is used in various industrial processes such as in the manufacture of lubricants, plastics, rubbers, dyes, synthetic fibres, etc. However, it has non-industrial uses too which are limited due to the reason benzene is toxic and carcinogenic. The different uses of Benzene are mentioned below.
- Benzene is used in the preparation of phenol. It is also used to prepare aniline used in dyes and in dodecylbenzene used for the detergents.
- In early times, benzene was used in degreasing of metal.
- It is used for manufacturing of nylon fibres.
- The main use of benzene is that it is used in the manufacture of other chemicals such as ethylbenzene, cyclohexane, cumene, nitrobenzene, alkylbenzene, etc.
Health Effects of Benzene
Acute exposure to benzene produces toxic effects on the central nervous system; however, in order to evaluate the chronic effects, consideration must be given to the myelotoxic and possible chromosome damaging and leukemogenic effects of benzene. The time required for expression of chlorine benzene toxicity indicates a vast difference in individual sensitivity.
Most cases of severe benzene intoxication have been reported in workers exposed to rather high concentrations of benzene under somewhat unhygienic working conditions. It is probable that all cases reported as “Leukemia associated with benzene exposure” have resulted from exposure rather high concentrations of benzene and other chemicals.
Frequently Asked Questions
Who discovered the structure of benzene?
Friedrich August Kekule.
List any 3 food items that contain benzene
Pineapple Crush, Giant Light Cranberry Juice Cocktail, Aquacal Strawberry Flavored Water Beverage
What are the three important steps to produce benzene industrially?
The following three chemical processes contribute equally to the production of benzene at an industrial level:
- Catalytic reforming
- Toluene hydrodealkylation
- Steam cracking
Briefly explain the shape of benzene
Benzene has a ring structure which consists of six carbon atoms that are bonded in a planar or flat hexagon ring.
Is C6H6 toxic to humans?
The Department of Health and Human Services has stated that C6H6 can cause cancer (a carcinogen).
Is benzene a solvent?
Benzene is a widely used solvent and occurs in gasoline, car emissions and cigarette smoke. Historically, high-level benzene contamination has been widespread and, because of its carcinogenic effects, benzene contamination has typically become a matter of considerable concern.
What happens if you smell benzene?
Acute (short-term) inhalation exposure of humans to benzene may cause drowsiness, dizziness, headaches, as well as eye, skin, and respiratory tract irritation, and, at high levels, unconsciousness.
What is the most characteristic reaction of benzene?
Benzene and it’s derivatives is much more stable than expected. The extra stability means that benzene will less readily undergo addition reactions. The more loosely held electrons are open to attack by electrophiles. Hence, the characteristic reaction of benzene is electrophilic substitution reaction.
Read more:


Comments