What is Atomic Number and Mass Number?
Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom (A-Z) corresponds to the total number of subatomic particles present in the atom. The mass number reports the mass of the atom’s nucleus in atomic mass units (amu).
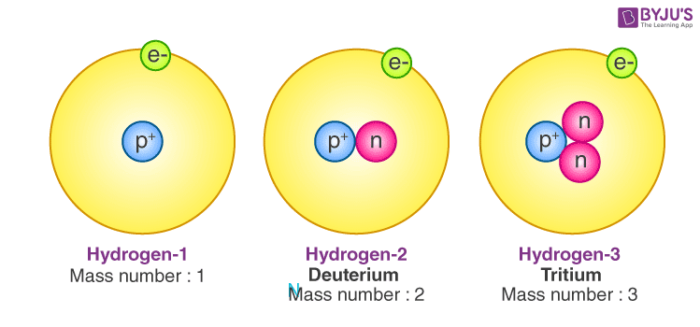
Isotopes are atoms with the same atomic number but different neutron numbers and thus different mass numbers. A little more than three-quarters of naturally occurring elements exist as a mixture of isotopes, and the element’s standard atomic weight is determined by the average isotopic mass of an isotopic mixture for an element called the relative atomic mass in a defined environment. Historically, scientists in the nineteenth century were able to measure the atomic weights of elements.
Table of Contents
- Atomic number
- Recommended Videos
- What is Atomic Number?
- What is the Mass Number?
- History of Atomic Number
- Examples of Atomic Number
- Atomic Number Orbital Energy Levels
- Difference between Valency, A and Z
- Solved Example
- Frequently Asked Questions – FAQs
Atomic Number
The atomic number of an element is determined by the number of protons in it, and it is used to differentiate one element from another. The mass number of an element is determined by the number of protons and neutrons combined.
The modern periodic table is arranged in such a way that all the elements have an increasing atomic number, and subsequently, increasing mass number. But do you know what mass number, or even what atomic number is?
Well, as you know, an atom consists of electrons, protons and neutrons. The number of electrons in the outermost shell gives us the valency of the atom. Similarly, the number of protons and neutrons are associated with the atomic number and mass number of the atom.
Recommended Videos
Introduction to Atomic and Molecular Weight

The Periodic Table

Let’s look at the difference between the two terms.
What is Atomic Number?
- The total number of protons in the nucleus of an atom gives us the atomic number of that atom.
- It is represented with the letter ‘Z.’
- All the atoms of a particular element have the same number of protons, and hence the same atomic number.
- Atoms of different elements have different atomic numbers.
- For example, all carbon atoms have the atomic number of 6, whereas all atoms of Oxygen have 8 protons in their nucleus.
What is Mass Number?
- The number of protons and neutrons combine to give us the mass number of an atom.
- It is represented using the letter ‘A.’
- As both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.
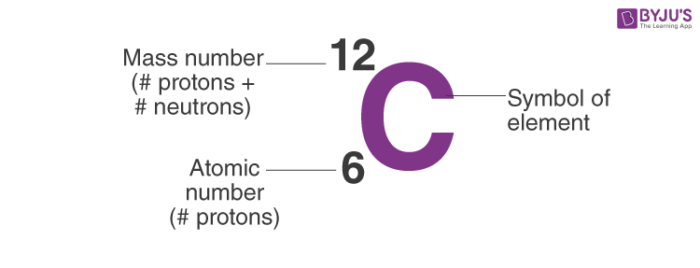
- For example, an atom of carbon has 6 protons and 6 neutrons. Thus, its mass number is 12.
- While the number of protons remains the same in all atoms of an element, the number of neutrons can vary. Thus, atoms of the same element can have different mass numbers, and these are called isotopes.
- The weight of an electron is almost negligible. Thus, the atomic mass of an atom is almost the same as its mass number.
History of Atomic Number
We take the existence of atoms for granted – yet for centuries no one believed in them. Around 2500 years ago a Greek philosopher called Leucippus and his pupil Demokritos put forward the idea that the universe is made up of tiny indivisible particles, which they called atoms. Unfortunately the great Greek philosopher Aristotle did not agree with them. As Aristotle views were accepted throughout Europe for almost 2000 years the idea of atoms was shelved for centuries to come.
Atoms are the building blocks of matter. They combine in numerous patterns and form different substances. All atoms except the common form of hydrogen contain protons, neutrons and electrons. The atomic number of an element is equal to the number of protons in its nucleus. In a neutral atom, the number of protons equals the number of electrons in shells which is the energy level around the nucleus.
Isotopes are atoms with the same atomic number but distinct neutron numbers, and hence distinct mass numbers. The average isotopic mass of an isotopic mixture for an element in a defined environment on Earth determines the element’s standard atomic weight. A little more than three-quarters of naturally occurring elements exist as a mixture of isotopes, and the average isotopic mass of an isotopic mixture for an element in a defined environment on Earth determines the element’s standard atomic weight.
Examples of Atomic Number
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom.
Atomic number = Number of protons
For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
Atomic Number Orbital Energy Levels
When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Orbitals are the name for these sections. Sublevels are made up of orbitals with the same energy. A maximum of two electrons can be found in each orbital.
The most common way of showing the arrangement of electrons in an atom is to draw diagrams like those shown in the diagram.
To write down the numbers of electrons in each energy level. The atomic number of an element tells us how many electrons there are in the atoms. For example, the atomic number of carbon is 6 giving us six electrons as 2,4. So an atom with the atomic number 12 has an electronic structure 2, 8, 2, with two electrons in the inner energy level, then eight in the next energy level and two in the outer highest energy level. The simplest way to understand these arrangements is to look at lots of examples of them.
Difference between Valency, A and Z:
When we want to find out the valency, we look at electrons only in the outermost shell of the atom. But when we want to know the atomic number or the mass number, we look at the total number of protons and neutrons.
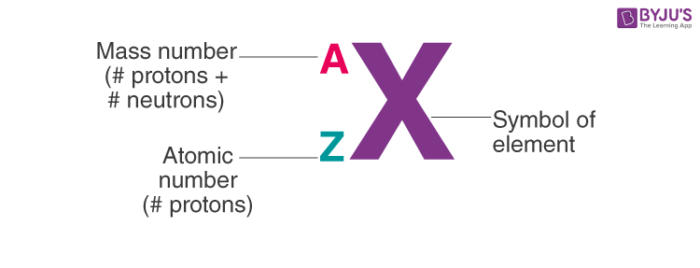
1. Notation of Atom
To write the notation of an atom, we need to know the symbol of the element, the atomic number and the mass number. The mass number of the atom goes above the symbol and the atomic number is written as a subscript.
So, the notation of Carbon is:
2. Calculating Number of Neutrons:
If we know the number of protons and the mass number of an element, we can also calculate the number of neutrons simply by subtracting its atomic number from its mass number.

Solved Example
Question:
An atom has an atomic number of 9 and a mass number of 19.
- Determine the number of protons present
- Determine the number of neutrons present
- Determine the number of electrons present
Solution:
- There are 9 protons because the atomic number is always equal to the number of protons present.
- There are 10 neutrons because the number of neutrons is always obtained by subtracting the atomic number from the mass number.
(protons + neutrons) – protons = neutrons - There are 9 electrons because the number of protons and the number of electrons are always the same in an atom.
Frequently Asked Questions – FAQs
What is the highest atomic number?
With atomic number 118, oganesson (symbol Og) is the highest atomic number element discovered or synthesised. This is a “magic” number in the sense that it completes the seventh row of the periodic table of elements.
Who discovered the atomic number?
The number of protons (positive charges) in the nucleus of an atom is given by its atomic number. This term was first introduced by Henry Gwyn-Jefferies Moseley.
How do you find atomic mass from the atomic number and the number of neutrons?
Add the mass of protons and neutrons to calculate the atomic mass of a single atom of an element. The number of protons and the number of neutrons together determine the mass number of an element: mass amount = protons + neutrons.
Why is the atomic number denoted by Z?
The atomic number symbol, Z, stands for “Zahl,” meaning German number. The symbol Z denoted an element’s place in the periodic table before 1915.
What is the mass number?
The complete amount in the protons and neutrons in the nucleus of an atom. For example, nitrogen has seven protons in its nucleus and seven neutrons, supplying it with 14 masses.
What is an atomic number and mass number?
The number of protons and neutrons combines to give us the mass number of an atom. It is represented using the letter ‘A.’ The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. It is represented using the letter ‘Z.’
To know more about the number of protons, neutrons and isotopes, download BYJU’S – The Learning App.


Very helpful
Good
its very helpfull . thanks buyjus for such a good explanation and for extra questions
What is the formula for a mass number of an atom?
Number of protons + number of electrons. Number of otons+ number of neutrons. b) d) Number of neutrons + number of electrons. None of these.
The number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. Click here to learn more about mass number.